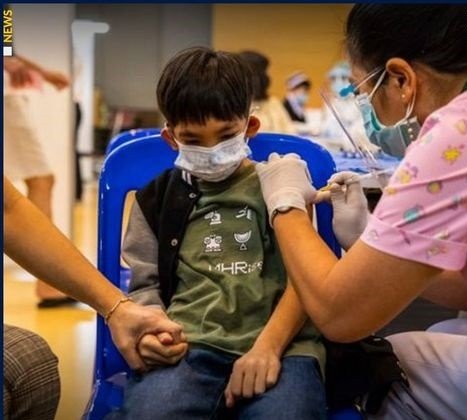
The Thai Food and Drug Administration (FDA) will soon start reviewing proposals for the emergency use of three COVID-19 vaccines for young children.
It is now awaiting safety and efficacy related data from manufacturers.
The Department of Disease Control (DDC) said the Thai FDA is now awaiting the submission of safety and efficacy data from Sinovac, Sinopharm, and Pfizer to extend the emergency use of these COVID-19 vaccines to young children.
DDC Director-General Dr. Opart Karnkawinpong said the vaccines, which are now available in Thailand, can immediately be administered for children after receiving the emergency authorization from the Thai FDA, after the review of relevant data.
Studies have been conducted for the use of the Sinopharm vaccine in younger children both in Thailand and abroad, while Malaysia and Indonesia have previously authorized the use of the Sinovac vaccine for children aged 12-18 years old.
Meanwhile, children aged 12-18 years old in Thailand are being offered the Pfizer jab, which is currently the only vaccine authorized by the Thai FDA for use in adolescents.
Pfizer-BioNTech this week received authorization from the U.S. FDA to use its vaccine among children aged 5-11 years old.
The DDC chief said the drug regulator is also waiting for information on adequate vaccine dosage for children from the manufacturers, which could be different from the recommended dosages for adults.
The Thai government is currently in talks with Pfizer to procure 30 million more doses, with an option to receive an updated or pediatric version of the jabs in future deliveries.
The government has also placed orders for 60 million doses of AstraZeneca jabs for next year.