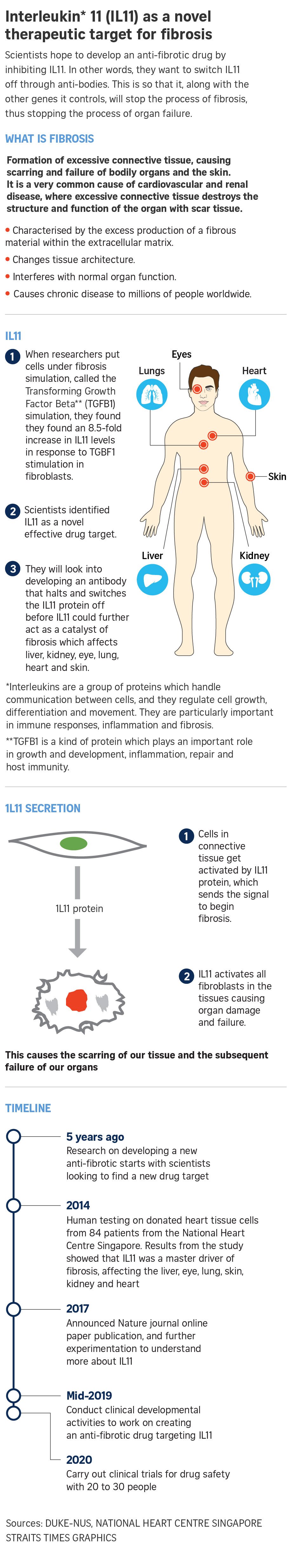
Protein discovered to be main driver of kidney, heart failure. A team of researchers from the Duke-NUS Medical School have made a breakthrough discovery: a protein called interleukin 11 (IL11) is the main protein activating and speeding up the processes of kidney and heart failure.
The researchers, led by principal investigator Professor Stuart Cook, experimented on the donated heart tissue samples of 84 patients from the National Heart Centre Singapore (NHCS) in 2016 for their research, which started five years ago.
They were trying to find a new gene to target that contributes to fibrosis, in which connective tissue is produced in excess, leading to organ failure in the liver, eye, lungs, skin, heart and the kidney.
Previously, the protein believed to be the main culprit was Transforming Growth Factor Beta (TGFB1), but the removal or reversal of the protein sometimes spelled adverse side effects in patients, leading to cancers or chest infections.
Prof Cook, the director of the Duke-NUS Programme in Cardiovascular and Metabolic Disorders, and his team at the NHCS looked at the samples cell by cell and gene by gene, and found that the interleukin 11 protein responded more than any other gene in the human body, showing up to 8.5 times more than normal when going through fibrosis.
The revelation was unprecedented, breaking down all previous misconceptions of the protein being harmless and dormant in the process of fibrosis.
Prof Cook even cited a previous study in 2010 that identified IL11 as antifibrotic, the complete opposite finding of his new results.
“Currently, over 225 million people suffer from a heart or kidney failure,” said Professor Terrance Chua, medical director of the NHCS. There is currently no treatment to prevent fibrosis, he added.
More Singaporeans, especially, suffer from the three most common diseases that lead to heart failure – coronary heart disease, hypertension and diabetes – as compared to other Asians, American and Europeans. This could be due to genetic reasons, said Prof Cook, who is also the Tanoto Foundation Professor of Cardiovascular Medicine.
The novel discovery, however, has the potential to change things around.
“Basically (IL11 is) a switch that makes TGFB1 fibrotic…but if we turn off IL11, TGFB1 doesn’t work anymore,” said Prof Cook.
“If we can turn IL11 and its receptor off in any way, we expect that it will improve fibrosis.”
Along with Professor Sebastian Schaefer, he set up a biotechnology company called Enleofen Bio Pte Ltd earlier this year, licensing their research exclusively to the company.
The firm will work with UK- and US-based bio pharmaceutical company Abzena to develop an antibody that inhibits IL11, according to Professor Cook, by mid-2019.
The two firms hope to carry out clinical trials on 20 to 30 people by 2020.
The Duke-NUS and NHCS findings have been published online in Nature as of Tuesday





