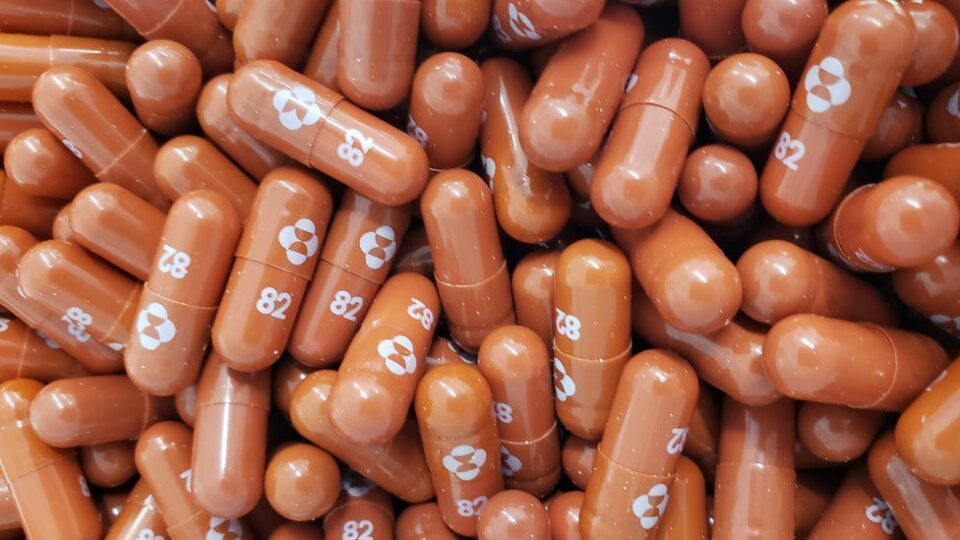
The first pill designed to treat symptomatic Covid has been approved by the UK medicines regulator.
The tablet – molnupiravir – will be given twice a day to vulnerable patients recently diagnosed with the disease.
In clinical trials the pill, originally developed to treat flu, cut the risk of hospitalisation or death by about half.
Health secretary Sajid Javid said the treatment was a “gamechanger” for the most frail and immunosuppressed.
In a statement he said: “Today is a historic day for our country, as the UK is now the first country in the world to approve an antiviral that can be taken at home for Covid.”
First oral treatment
Molnupiravir, developed by the US drug companies Merck, Sharp and Dohme (MSD) and Ridgeback Biotherapeutics, is the first oral antiviral medication for Covid which can be taken as a pill rather than injected or given intravenously
It targets an enzyme that the virus uses to make copies of itself, introducing errors into its genetic code. That should prevent it from multiplying, so keeping virus levels low in the body and reducing the severity of the disease.
Merck said that approach should make the treatment equally effective against new variants of the virus as it evolves in the future.
The UK regulator, the MHRA, said the tablet had been authorised for use in people who have mild to moderate Covid and at least one risk factor for developing severe illness such as obesity, old age, diabetes or heart disease.
The organisation’s chief executive, June Raine, described the treatment as “another therapeutic to add to our armoury against Covid-19”.
“It is the world’s first approved antiviral for this disease that can be taken by mouth rather than administered intravenously,” she said.
“This is important, because it means it can be administered outside of a hospital setting, before Covid-19 has progressed to a severe stage.”
The UK has initially ordered 480,000 courses of molnupiravir to be delivered by the end of the year, along with 250,000 courses of a similar experimental drug currently being developed by US drug company Pfizer.
The UK government has not revealed how much the contract is worth.
But the US government has made an advance purchase of 1.7 million courses of the drug at a cost of roughly $1.2 billion, or roughly $700 (£513) for each course.
Other countries including Australia, Singapore and South Korea have made purchase agreements.
Professor Stephen Powis, national medical director for NHS England, said: “The NHS stands ready to support the planned study on molnupiravir and other antivirals in patients at higher risk of complications, and to provide wider roll out if it is shown to be clinically and cost effective in reducing hospitalisations and death.”
Clinical trials
Earlier clinical trials of molnupiravir on 775 patients who had recently caught Covid found:
- 7.3% of those given the drug were hospitalised
- that compares with 14.1% of patients who were given a placebo or dummy pill
- there were no deaths in the molnupiravir group, but eight patients who were given a placebo in the trial later died of Covid
The data was published in a press release and has not yet been peer-reviewed.
Trial results suggest molnupiravir needs to be taken soon after symptoms develop to have an effect. An earlier study in patients who had already been hospitalised with severe Covid was halted after disappointing results.
Merck is the first company to report trial results of a pill to treat Covid, but other companies are working on similar treatments.
Its US rival Pfizer has started trials of two different antiviral tablets, while Swiss company Roche is working on a similar medication.